Li-ion is a low-maintenance battery, an advantage that most other chemistries cannot claim. The battery has no memory and does not need exercising (deliberate full discharge) to keep it in good shape. Self-discharge is less than half that of nickel-based systems and this helps the fuel gauge applications. The nominal cell voltage of 3.60V can directly power mobile phones, tablets and digital cameras, offering simplifications and cost reductions over multi-cell designs. The drawbacks are the need for protection circuits to prevent abuse, as well as high price.
Types of Lithium-ion Batteries
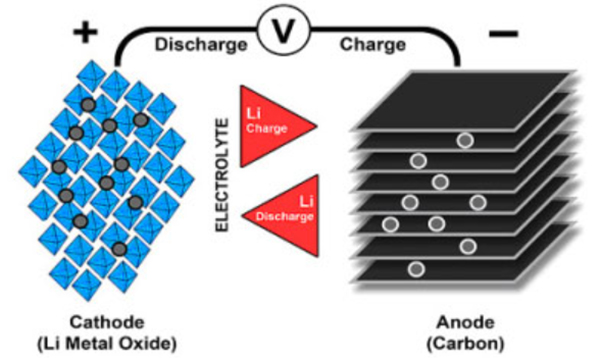
Figure 1 illustrates the process.
Li-ion is a low-maintenance battery, an advantage that most other chemistries cannot claim. The battery has no memory and does not need exercising (deliberate full discharge) to keep it in good shape. Self-discharge is less than half that of nickel-based systems and this helps the fuel gauge applications. The nominal cell voltage of 3.60V can directly power mobile phones, tablets and digital cameras, offering simplifications and cost reductions over multi-cell designs. The drawbacks are the need for protection circuits to prevent abuse, as well as high price.
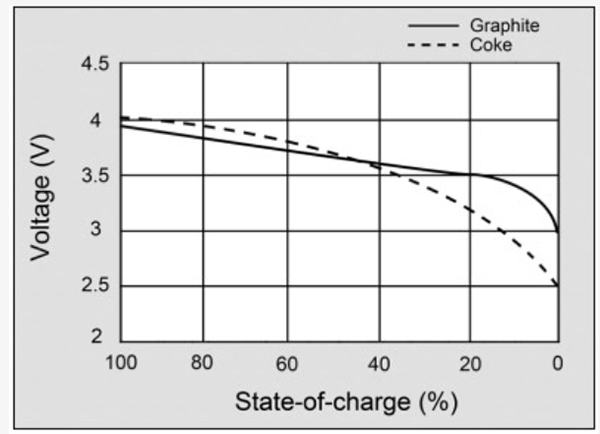
Sony’s original lithium-ion battery used coke as the anode (coal product). Since 1997, most Li ion manufacturers, including Sony, shifted to graphite to attain a flatter discharge curve. Graphite is a form of carbon that has long-term cycle stability and is used in lead pencils. It is the most common carbon material, followed by hard and soft carbons. Nanotube carbons have not yet found commercial use in Li-ion as they tend to entangle and affect performance. A future material that promises to enhance the performance of Li-ion is graphene.
Figure 2 illustrates the voltage discharge curve of a modern Li-ion with graphite anode and the early coke version.
Several additives have been tried, including silicon-based alloys, to enhance the performance of the graphite anode. It takes six carbon (graphite) atoms to bind to a single lithium ion; a single silicon atom can bind to four lithium ions. This means that the silicon anode could theoretically store over 10 times the energy of graphite, but expansion of the anode during charge is a problem. Pure silicone anodes are therefore not practical and only 3–5 percent of silicon is typically added to the anode of a silicon-based to achieve good cycle life.
Using nano-structured lithium-titanate as an anode additive shows promising cycle life, good load capabilities, excellent low-temperature performance and superior safety, but the specific energy is low and the cost is high.
Experimenting with cathode and anode material allows manufacturers to strengthen intrinsic qualities, but one enhancement may compromise another. The so-called “Energy Cell” optimizes the specific energy (capacity) to achieve long runtimes but at lower specific power; the “Power Cell” offers exceptional specific power but at lower capacity. The “Hybrid Cell” is a compromise and offers a little bit of both.
Manufacturers can attain a high specific energy and low cost relatively easily by adding nickel in lieu of the more expensive cobalt, but this makes the cell less stable. While a start-up company may focus on high specific energy and low price to gain quick market acceptance, safety and durability cannot be compromised. Reputable manufacturers place high integrity on safety and longevity.
Most Li-ion batteries share a similar design consisting of a metal oxide positive electrode (cathode) that is coated onto an aluminum current collector, a negative electrode (anode) made from carbon/graphite coated on a copper current collector, a separator and electrolyte made of lithium salt in an organic solvent. More informations, pls go with teda battery.com.
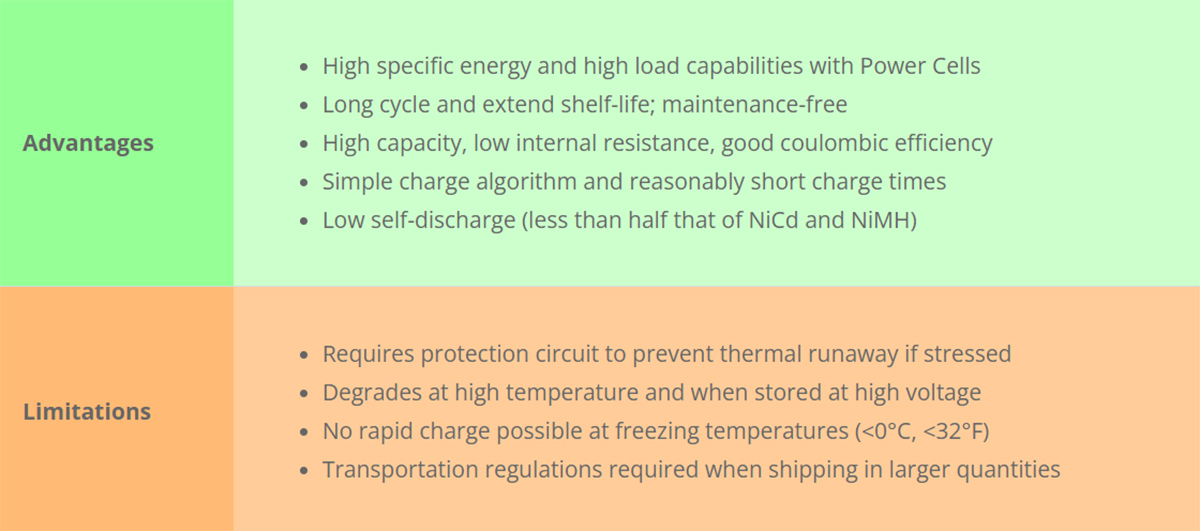
Table 3 summarizes the advantages and limitations of Li-ion.
Post time: Jun-26-2022

